Glutamine Metabolism and the PI3K/mTOR Pathway
Cancer metabolism has become an important field in cancer research and metabolic reprogramming in cancer cells has acquired the status of a core hallmark of cancer. One of the unique metabolic characteristics of cancer cells is their addiction to glutamine. Due to their fast proliferation rate, many cancers exceed their capacity to use glucose in support of bioenergetics and macromolecular synthesis. Therefore, using glutamine as a secondary nutrient source becomes essential. Glutamine plays an important role as a nitrogen donor in nucleotide and amino acid biosynthesis. In addition, it provides fuel for the TCA cycle, plays a required role in the uptake of essential amino acids and is involved in the control of reactive oxygen species.
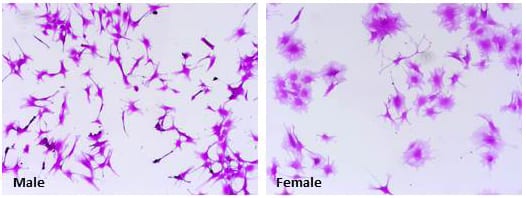
Sex differences in metabolism are well reported in numerous developmental stages and sex differences in growth are most likely the result of sex differences in metabolic pathways. However, sex differences in cancer metabolism remain largely unexplored. Currently we are investigating sex differences in glioblastoma metabolism, with a focus in glutamine metabolism. We hypothesize that male glioblastoma exhibit a higher activity in glutamine metabolism which contributes to their aggressive tumorigenic phenotype we observed in the past. Understanding the differences in male and female metabolism in glioblastoma will help us understand the molecular mechanisms that underlie sex differences in glioblastoma. Targeting cancer metabolism is currently a major focus in cancer research. Therefore, our findings could have direct impact on treatment strategies for male and female glioblastoma patients.
A major regulator of metabolic pathways is the PI3K/mTOR pathway. It is involved in the regulation of glycolysis, nucleotide synthesis, protein biosynthesis, and glutamine metabolism. Therefore, we are currently investigating whether sex differences in metabolism underlie sex differences in the PI3K/mTOR pathway. Recently, PI3K/mTOR pathway inhibitors have been exploited as potential therapeutics in GBM, and numerous PI3K/mTOR pathway inhibitors are currently under clinical investigation. Therefore, our findings could have a crucial impact on current and future clinical investigations.